Table of Contents
Procedure of Trial Intervention
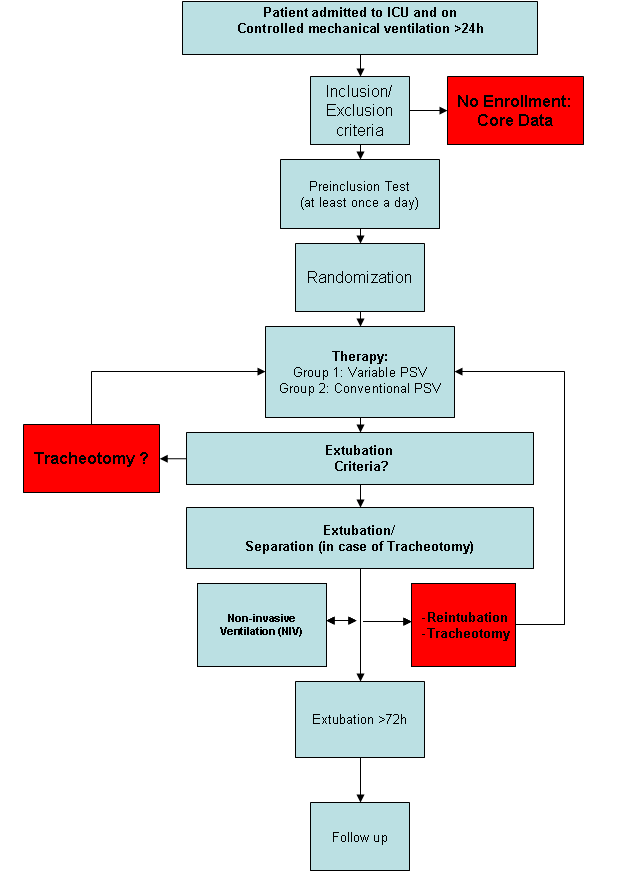
Figure 1 depicts the plan of treatment. Two mechanical ventilators “Dräger Evita Infinity® V500” with the option “variable pressure support” will be available at each participating study site, since this is the the only ventilator with able to provide variable PSV. At the moment of randomization, at least one Dräger Evita Infinity® V500 must be ready for use. All Patients admitted to a participating ICU and controlled mechanically ventilated for more than 24 hours are eligible for the study and will be checked for inclusion criteria. If the patient does not full-fill those criteria, only “core data” is recorded. “Core data” involves basic demographic data and the reason of not participating in the study.
Preinclusion Test
Upon informed consent of a next-of-kin and/or a legal representative according to local regulations the patient is monitored for readiness to perform the pre-inclusion test. If the pre-inclusion test is passed, the patient is randomly assigned to one of the assisted mechanical ventilation groups. If the patient fails the pre-inclusion test, mechanical ventilation is conducted according to local clin-ical practice. The pre-inclusion test is repeated as early as possible at discretion of the treating physician. The pre-inclusion test has a total duration of 1 hour. During the pre-inclusion test the patient will be ventilated in PSV Mode according to the following settings:
- targeted mean VT = 6-8 ml/kg
- pressure support variability = 0%
- maximal peak pressure = 40 cmH2O
- flow trigger = 2 l/min
- inspiratory cycling-off = 25% of peak flow
- PEEP and FIO2 for SaO2 ≥ 92%, whereby PEEP ≥ 5 cmH2O
If the patients shows hemodynamic or respiratory signs of distress, the test wil be stopped ahead of time. The pre-inclusion test is successful if:
- Temperature ≤ 39 °C
- PaO2/FIO2 Ratio ≥ 150 mmHg with PEEP ≤ 16 cmH2O
- pH ≥ 7.30
- RR ≤ 40/min
- Heart rate 40 to 130/min
- Systolic blood pressure 80 to 160 mmHg
- Maximal 0.1 mcg/kg/min noradrenaline, 0.1 mcg/kg/min adrenaline, 2 mcg/kg/min dopa-mine, 2 mcg/kg/min dobutamine
- no hemodynamic relevant acute cardiac arrhythmia
Randomization
If the pre-inclusion test is passed, the patient is randomly assigned to one of the two groups:
- variable PSV (Group 1)
- conventional PSV (Group 2)
Study Therapy
Ventilator settings
In Group 1 the following mechanical ventilation settings will be used:
- targeted mean VT = 6-8 ml/kg
- pressure support variability as high as possible without exceeding the maximal inspiratory pressure
- maximal inspiratory pressure = 40 cmH2O
- flow trigger = 2 l/min
- inspiratory cycling-off at 25% of peak flow
- PEEP and FIO2 for SaO2 ≥ 92%, whereby PEEP ≥ 5 cmH2O
In Group 2 the following mechanical ventilation settings will be used:
- targeted mean VT = 6-8 ml/kg
- pressure support variability = 0%
- maximal peak pressure = 40 cmH2O
- flow trigger = 2 l/min
- inspiratory cycling-off = 25% of peak flow
- PEEP and FIO2 for SaO2 ≥ 92%, whereby PEEP ≥ 5 cmH2O
Ventilator adjustment
The patient will be ventilated with the assigned mechanical ventilation mode until extubation. In case of interruption of the assigned ventilation mode, for example due to transport, the assigned mechanical ventilation mode has to be resumed as soon as possible. The mechanical ventilator settings are modified stepwise until the patient fulfills the extubation cri-teria. Adjustment of pressure support until extubation:
- pressure support is gradually adjusted by steps of up to 5 cmH2O (up and downwards)
- PEEP is decreased by steps of up to 5 cmH2O
- PEEP and FIO2 for SaO2 ≥ 92%, whereby PEEP ≥ 5 cmH2O
Extubation
As the ventilator settings are stepwise reduced to reach extubation criteria, the patients condition is continuously monitored. As soon as the patient fulfills the following criteria for a time period of 30 minutes, extubation is possible. Extubation Criteria (during 30 min):
- RASS ≥ -3
- Behavioral Pain Scale (BPS) ≤ 5 or Visual Analogue Scale (VAS) for pain ≤ 3
- Raising hands or legs against gravity
- Temperature ≥ 36°C and ≤ 39°C
- Ability to cough to clear secretions after deflating cuff
- Respiratory rate 8 to 30/min
- PaO2/FIO2 ≥ 200 mmHg (≥150 mmHg if COPD)
- Pressure support ≤ 8 cmH2O
- PEEP ≤ 8 cmH20
- Systolic blood pressure 80 to 160 mmHg
- Heart rate 40 to 130/min
- No hemodynamic relevant arrhythmia
Successful extubation - End of Intervention
If the patient needs to be reintubated within 72 hours, the extubation is defined as “not successful” and the assigned study mechanical ventilation mode will be reinitiated as soon as possible again. If the patient has to be tracheotomized during the study therapy, assisted mechanical ventilation will be resumed as soon as possible via the tracheal cannula according to the assigned therapy group.
Successful extubation is defined as
- Extubation without reintubation < 72h after extubation
- (in tracheotomized patients) separation from ventilator without reconnection < 72h after separation